Chemical compounds can be categorized into various types based on their composition, structure, and properties. Here’s a list of some common types of chemical compounds:
Inorganic Compounds: These compounds do not contain carbon-hydrogen (C-H) bonds. Examples include water (H2O), sodium chloride (NaCl), and sulfuric acid (H2SO4).
Examples :
- Water (H2O)
- Sodium Chloride (NaCl)
- Calcium Carbonate (CaCO3)
- Potassium Nitrate (KNO3)
- Ammonium Sulfate ((NH4)2SO4)
- Hydrochloric Acid (HCl)
- Sulfuric Acid (H2SO4)
- Carbon Dioxide (CO2)
- Nitrogen Gas (N2)
- Iron Oxide (Fe2O3)
- Carbonates (e.g., Na2CO3)
- Silicon Dioxide (SiO2)
- Phosphoric Acid (H3PO4)
- Aluminum Oxide (Al2O3)
These are just a few examples of inorganic compounds, and there are many more with diverse properties and applications in various industries.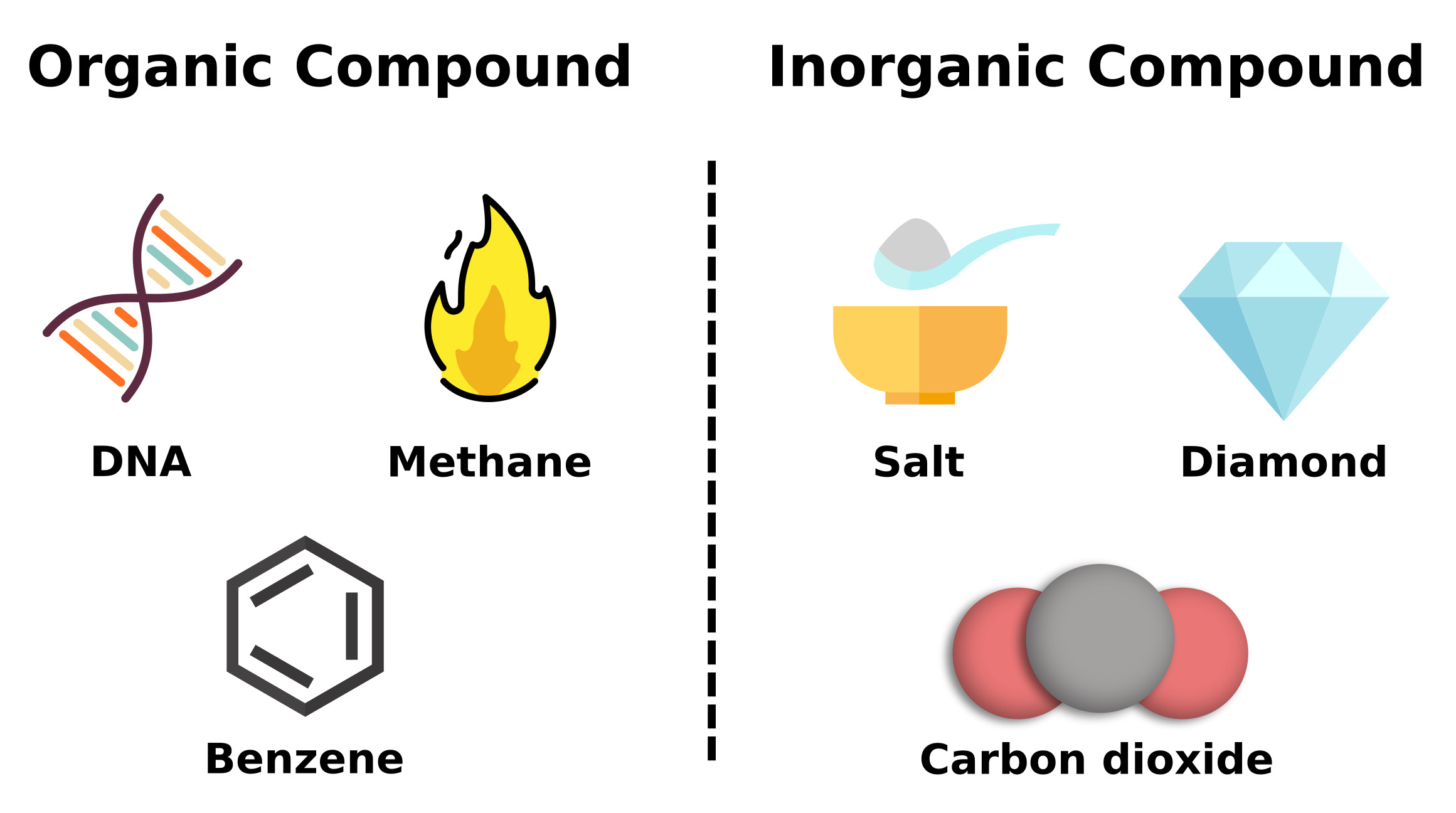
Organic Compounds: These compounds contain carbon atoms bonded to hydrogen and other elements like oxygen, nitrogen, sulfur, and halogens. Organic compounds are the basis of organic chemistry and include molecules such as methane (CH4), ethanol (C2H5OH), and glucose (C6H12O6).
Examples :
- Methane (CH4)
- Ethanol (C2H5OH)
- Glucose (C6H12O6)
- Acetic Acid (CH3COOH)
- Benzene (C6H6)
- Aspirin (Acetylsalicylic Acid)
- DNA (Deoxyribonucleic Acid)
- Proteins
- Lipids
- Caffeine (Trimethylxanthine)
- Chlorophyll
- Adenosine Triphosphate (ATP)
- Amino Acids
- Fatty Acids
- Vitamin C (Ascorbic Acid)
These examples represent just a small fraction of the vast diversity of organic compounds found in nature and synthesized for various purposes in chemistry, biology, and industry.
Ionic Compounds: Ionic compounds are formed by the transfer of electrons between atoms, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions).
Common examples include table salt (NaCl) and calcium chloride (CaCl2).
Example :
- Sodium Chloride (NaCl)
- Potassium Bromide (KBr)
- Calcium Carbonate (CaCO3)
- Magnesium Oxide (MgO)
- Aluminum Sulfate (Al2(SO4)3)
- Potassium Nitrate (KNO3)
- Barium Chloride (BaCl2)
- Lithium Fluoride (LiF)
- Sodium Oxide (Na2O)
- Calcium Phosphate (Ca3(PO4)2)
- Ammonium Chloride (NH4Cl)
- Silver Nitrate (AgNO3)
- Iron(III) Sulfide (Fe2S3)
These examples demonstrate the wide range of ionic compounds with various applications in chemistry, industry, and everyday life. Ionic compounds typically have high melting and boiling points and conduct electricity when dissolved in water or in molten form due to the movement of ions.
Covalent Compounds (Molecular Compounds): Covalent compounds are formed by the sharing of electrons between atoms. These compounds can be simple molecules like oxygen (O2), hydrogen (H2), and carbon dioxide (CO2).
Example :
- Water (H2O)
- Methane (CH4)
- Carbon Dioxide (CO2)
- Ammonia (NH3)
- Ethanol (C2H5OH)
- Methanol (CH3OH)
- Carbon Tetrachloride (CCl4)
- Oxygen Gas (O2)
- Hydrogen Peroxide (H2O2)
- Sulfur Hexafluoride (SF6)
- Nitrogen Gas (N2)
- Carbon Monoxide (CO)
- Hydrogen Cyanide (HCN)
- Sulfur Dioxide (SO2)
- Acetone (CH3COCH3)
These examples demonstrate the diversity of covalent compounds and their importance in various chemical reactions, biological processes, and industrial applications. Covalent compounds tend to have lower melting and boiling points compared to ionic compounds and often do not conduct electricity in their pure molecular form
Acids: Acids are substances that release hydrogen ions (H+) when dissolved in water. Examples include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).
Example :
Strong Acids:
- Hydrochloric Acid (HCl)
- Sulfuric Acid (H2SO4)
- Nitric Acid (HNO3)
- Hydrobromic Acid (HBr)
- Perchloric Acid (HClO4)
Weak Acids:
- Acetic Acid (CH3COOH)
- Citric Acid (C6H8O7)
- Carbonic Acid (H2CO3)
- Phosphoric Acid (H3PO4)
- Formic Acid (HCOOH)
- Benzoic Acid (C6H5COOH)
- Oxalic Acid (H2C2O4)
- Tartaric Acid (C4H6O6)
These examples showcase a range of acids with different properties and applications, both in industry and in everyday life. Acids are known for their ability to react with bases to form salts and water in neutralization reactions, and they are an important class of chemicals in chemistry.
Bases: Bases are substances that release hydroxide ions (OH-) when dissolved in water. Common bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
Example :
Strong Bases:
- Sodium Hydroxide (NaOH)
- Potassium Hydroxide (KOH)
- Calcium Hydroxide (Ca(OH)2)
- Barium Hydroxide (Ba(OH)2)
- Lithium Hydroxide (LiOH)
Weak Bases:
- Ammonia (NH3)
- Aluminum Hydroxide (Al(OH)3)
- Magnesium Hydroxide (Mg(OH)2)
- Copper Hydroxide (Cu(OH)2)
- Zinc Hydroxide (Zn(OH)2)
- Sodium Bicarbonate (NaHCO3)
- Methylamine (CH3NH2)
These examples illustrate a range of bases with varying strengths and applications. Bases are often used to neutralize acids, and their properties are important in various chemical reactions and industrial processes.
Salts: Salts are compounds formed when an acid reacts with a base, resulting in the neutralization of the acid’s properties. Examples include sodium sulfate (Na2SO4) and calcium carbonate (CaCO3).
Example :
- Sodium Chloride (NaCl)
- Calcium Carbonate (CaCO3)
- Potassium Nitrate (KNO3)
- Magnesium Sulfate (MgSO4)
- Ammonium Chloride (NH4Cl)
- Potassium Chloride (KCl)
- Sodium Bicarbonate (NaHCO3)
- Iron(II) Sulfate (FeSO4)
- Sodium Carbonate (Na2CO3)
- Calcium Chloride (CaCl2)
- Potassium Hydroxide (KOH)
- Sodium Sulfate (Na2SO4)
These examples demonstrate the diversity of salts and their wide range of applications in various industries, including food, agriculture, chemistry, and manufacturing.
Oxides: Oxides are compounds that contain oxygen and another element. They can be basic (metal oxides), acidic (non-metal oxides), or amphoteric (both acidic and basic). Examples include carbon dioxide (CO2), silicon dioxide (SiO2), and iron oxide (Fe2O3).
Example :
Metal Oxides (Basic Oxides):
- Iron(III) Oxide (Fe2O3)
- Copper Oxide (CuO)
- Aluminum Oxide (Al2O3)
- Magnesium Oxide (MgO)
- Zinc Oxide (ZnO)
Non-Metal Oxides (Acidic Oxides):
- Carbon Dioxide (CO2)
- Sulfur Dioxide (SO2)
- Nitrogen Dioxide (NO2)
Acidic Anhydrides (Non-Metal Oxides):
- Sulfur Trioxide (SO3)
- Carbon Tetrachloride (CCl4)
Mixed Oxides:
- Silicon Dioxide (SiO2)
- Lead(II) Oxide (PbO)
- Titanium Dioxide (TiO2)
These examples illustrate the diverse range of oxides and their various applications in industry, chemistry, and everyday life. Oxides are essential compounds with significant roles in many chemical processes and materials.
Hydrocarbons: Hydrocarbons are organic compounds composed of hydrogen and carbon atoms. They can be further classified into alkanes (saturated hydrocarbons), alkenes (hydrocarbons with double bonds), and alkynes (hydrocarbons with triple bonds).
Example :
Alkanes (Saturated Hydrocarbons):
- Methane (CH4)
- Ethane (C2H6)
- Propane (C3H8)
- Butane (C4H10)
Alkenes (Unsaturated Hydrocarbons with Double Bonds):
- Ethene (C2H4)
- Propene (C3H6)
- Butene (C4H8)
Alkynes (Unsaturated Hydrocarbons with Triple Bonds):
- Ethyne (Acetylene, C2H2)
- Propyne (C3H4)
- Butyne (C4H6)
These hydrocarbon examples represent some of the simplest organic compounds, but hydrocarbons can become quite complex in larger molecules found in petroleum, plastics, and many organic chemicals. Hydrocarbons are crucial in the petrochemical industry and are the basis for the production of various fuels, lubricants, and synthetic materials.
Alcohols: Alcohols are organic compounds containing a hydroxyl (-OH) functional group. Examples include ethanol (C2H5OH) and methanol (CH3OH).
Examples :
Aliphatic Alcohols:
- Methanol (CH3OH)
- Ethanol (C2H5OH)
- Isopropanol (Isopropyl Alcohol, C3H8O)
- Butanol (C4H10O
Aromatic Alcohols:
- Phenol (C6H6O)
- Glycerol (C3H8O3)
Cyclic Alcohols (Cycloalcohols):
- Cyclohexanol (C6H12O)
- Menthol (C10H20O)
Secondary and Tertiary Alcohols:
- Isobutyl Alcohol (2-Methyl-1-propanol, C4H10O)
- Tert-Amyl Alcohol (2-Methyl-2-butanol, C5H12O)
These examples illustrate the diversity of alcohols and their wide range of applications in industry, medicine, and everyday life. Alcohols are versatile organic compounds with various physical and chemical properties depending on their structure and functional group.
Ethers: Ethers are organic compounds containing an oxygen atom bonded to two alkyl or aryl groups. An example is dimethyl ether (CH3OCH3).
Examples :
Simple Ethers:
- Dimethyl Ether (DME, CH3OCH3)
- Diethyl Ether (C2H5OC2H5)
- Methyl Ethyl Ether (CH3OC2H5)
Cyclic Ethers (Oxiranes):
A.Epoxide (Ethylene Oxide, C2H4O)
Aromatic Ethers:
A.Anisole (Methoxybenzene, C7H8O)
Polymeric Ethers (Polyether’s):
- Polyethylene Glycol (PEG)
- Polypropylene Glycol (PPG)
Heterocyclic Ethers:
- Tetrahydrofuran (THF, C4H8O)
- Dioxane (1,4-Dioxane, C4H8O2)
Crown Ethers:
1.18-Crown-6 (C12H24O6)
These examples showcase the versatility of ether compounds in various applications, including in the chemical industry, pharmaceuticals, and research. Ethers are known for their ability to act as solvents and for their use in some chemical reactions and organic synthesis processes.
Amines: Amines are organic compounds containing a nitrogen atom bonded to hydrogen or other organic groups. Examples include ammonia (NH3) and methylamine (CH3NH2).
Example :
Primary Amines (R-NH2):
- Methylamine (CH3NH2)
- Ethylamine (C2H5NH2)
- Aniline (C6H5NH2)
Secondary Amines (R2-NH):
- Dimethylamine (CH3NHCH3)
- Diethylamine (C2H5NHCH3)
Tertiary Amines (R3-N):
- Trimethylamine (N(CH3)3)
- Triethylamine (N(C2H5)3)
Aromatic Amines:
- Aniline (C6H5NH2)
- Methylaniline (C6H5NHCH3)
Heterocyclic Amines:
- Pyridine (C5H5N)
- Piperidine (C5H11N)
These examples represent a range of amines with various structures and applications in industry, chemistry, and pharmaceuticals. Amines play a significant role in the synthesis of organic compounds and have various biological functions as well.
Ketones: Ketones are organic compounds containing a carbonyl group (C=O) bonded to two carbon atoms. Acetone (CH3COCH3) is a common example.
Example :
Simple Ketones:
- Acetone (Propanone, CH3COCH3)
- Butanone (Methyl Ethyl Ketone, MEK, CH3COCH2CH3)
- Cyclohexanone (C6H10O)
Aromatic Ketones:
- Acetophenone (C6H5C(O)CH3)
- Benzophenone (C13H10O)
Aliphatic Ketones:
- 2-Pentanone (Methyl Propyl Ketone, C5H10O)
- 3-Heptanone (C7H14O)
Ketones in Nature:
- Camphor (C10H16O)
- Furanone (C4H4O2)
Ketone Sugars:
1.Fructose (C6H12O6)
These examples illustrate the diversity of ketones and their applications in various industries, including chemicals, pharmaceuticals, and the food and fragrance sectors. Ketones play an important role in organic chemistry due to their reactivity in a wide range of chemical reactions.
Aldehydes: Aldehydes are organic compounds containing a carbonyl group bonded to at least one hydrogen atom. Formaldehyde (HCHO) is a simple aldehyde.
Example :
Simple Aldehydes:
- Formaldehyde (Methanal, CH2O)
- Acetaldehyde (Ethanal, CH3CHO)
Aromatic Aldehydes:
- Benzaldehyde (C6H5CHO)
- Vanillin (C8H8O3)
Aliphatic Aldehydes:
- Propionaldehyde (Propanal, C3H6O)
- Butyraldehyde (Butanal, C4H8O)
Ketone Aldehydes:
- Propanone (Acetone, CH3COCH3)
- Cinnamaldehyde (C9H8O)
- Citral (C10H16O)
These examples showcase the diversity of aldehydes and their applications in various industries, including food, perfumery, and the production of chemicals and pharmaceuticals. Aldehydes are known for their distinctive and often pleasant aromas, making them valuable in flavourings and fragrances.
Carboxylic Acids: Carboxylic acids are organic compounds characterized by the presence of a carboxyl group (–COOH), which consists of a carbonyl group (C=O) and a hydroxyl group (–OH) bonded to the same carbon atom. They are typically acidic and can donate a proton (H+) to water to form hydronium ions (H3O+). Here are some examples of carboxylic acids:
Examples :
Aliphatic Carboxylic Acids:
- Acetic Acid (Ethanoic Acid, CH3COOH)
- Formic Acid (Methanoic Acid, HCOOH)
- Butyric Acid (Butanoic Acid, C3H7COOH)
- Palmitic Acid (Hexadecenoic Acid, C15H31COOH)
Aromatic Carboxylic Acids:
- Benzoic Acid (C6H5COOH):
- Salicylic Acid (2-Hydroxybenzoic Acid, C7H6O3)
Dicarboxylic Acids:
- Oxalic Acid (Ethanedioic Acid, C2H2O4)
- Malonic Acid (Propanedioic Acid, C3H4O4)
Tricarboxylic Acids:
1.Citric Acid (C6H8O7)
Fatty Acids:
- Stearic Acid (Octadecanoic Acid, C18H36O2)
- Linoleic Acid (C18H32O2)
These examples highlight the diversity of carboxylic acids and their various applications in food, cosmetics, pharmaceuticals, and the chemical industry. Carboxylic acids are significant compounds in both natural and synthetic contexts, with a wide range of uses and properties.
Esters: Esters are organic compounds formed by the reaction between a carboxylic acid and an alcohol. Examples include ethyl acetate (CH3COOC2H5) and methyl salicylate (CH3OC6H4COOCH3).
Examples :
Fruit Esters:
- Ethyl Acetate (CH3COOC2H5)
- Isoamyl Acetate (Banana Oil, CH3COOCH2CH2CH(CH3)2)
Floral Esters:
- Methyl Salicylate (Wintergreen Oil, CH3COOC6H4OH)
- Geranyl Acetate (CH3COOC10H17)
Artificial Flavor Esters:
- Amyl Acetate (Pentyl Acetate, CH3COOC5H11)
- Butyl Butyrate (CH3CH2CH2COOCH2CH2CH2CH3)
Solvent Esters:
- Diethyl Phthalate (DEP, C12H14O4)
- Triacetin (Glyceryl Triacetate, C9H14O6)
Esters in Pharmaceuticals:
- Aspirin (Acetylsalicylic Acid, CH3COOC6H4COOH)
- Polyethylene Terephthalate (PET)
These examples demonstrate the versatility of esters and their wide range of applications in industry, food, cosmetics, and pharmaceuticals. Esters contribute to the Flavors and scents of many fruits and are important components of artificial Flavors and fragrances.
Lipids: Lipids are a diverse group of organic compounds, including fats, oils, and phospholipids, that are insoluble in water and important for energy storage and cell structure.
Example :
Fats and Oils (Triglycerides):
- Olive Oil
- Butter
- Coconut Oil
- Avocado
Phospholipids:
- Phosphatidylcholine
- Phosphatidylserine
Steroids:
- Cholesterol
- Hormones
Waxes:
1.Beeswax
Glycolipids:
1.Cerebrosides
Sphingolipids:
1.Sphingomyelin
Omega-3 Fatty Acids:
- Docosahexaenoic Acid (DHA)
- Eicosapentaenoic Acid (EPA)
These examples represent the diversity of lipids and their various roles in living organisms. Lipids are essential components of biological membranes, energy storage molecules, and signaling molecules, and they contribute to the taste and texture of many foods.
Polymers: Polymers are large molecules made up of repeating units called monomers. Examples include polyethylene, polypropylene, and DNA.
Example :
Natural Polymers:
- Cellulose
- Starch
- Proteins
- DNA (Deoxyribonucleic Acid)
- Rubber
Synthetic Polymers:
- Polyethylene (PE)
- Polypropylene (PP)
- Polyvinyl Chloride (PVC)
- Polystyrene (PS)
- Polyester
- Nylon
- Polyurethane (PU)
Biodegradable Polymers:
- Polylactic Acid (PLA)
- Polyhydroxyalkanoates (PHAs)
Silicones:
1.Silicone Polymers
Elastomers:
- Neoprene
- Silicone Elastomers
These examples illustrate the vast diversity of polymers and their applications in various industries, from packaging and textiles to healthcare and electronics. Polymers are integral to modern life and play a crucial role in a wide range of products and technologies.
Coordination Compounds: Coordination compounds are complexes in which metal ions are bonded to surrounding ligands through coordinate bonds. They have diverse applications in catalysis, medicine, and materials science.
Examples :
Simple Coordination Complexes:
- Tetraamminecopper(II) ion ([Cu(NH3)4]2+)
- Hexaaquachromium(III) ion ([Cr(H2O)6]3+)
Chelating Ligands:
- Ethylenediaminetetraacetatoiron(III) ion ([Fe(EDTA)]-)
- Cisplatin ([Pt(NH3)2Cl2])
Complex Ions in Biological Systems:
- Hemoglobin
- Chlorophyll
Mixed Ligand Complexes:
1.Cyanocobalamin (Vitamin B12)
Catalysts and Coordination Compounds:
- Wilkinson’s Catalyst ([RhCl(PPh3)3])
- Prussian Blue ([Fe4[Fe(CN)6]3])
These examples highlight the diverse range of coordination compounds and their applications in chemistry, biology, medicine, and industry. Coordination chemistry plays a crucial role in understanding the behaviour of transition metals and their interactions with ligands, making it a fundamental field in chemistry.
Heterocyclic Compounds : Heterocyclic compounds are organic compounds that contain at least one ring structure with carbon atoms and at least one atom from another element (usually nitrogen, oxygen, or sulfur) within the ring. These compounds are diverse and have a wide range of applications in chemistry, biology, and pharmaceuticals. Here are some examples of heterocyclic compounds:
Examples :
Pyridines:
- Pyridine (C5H5N)
- Nicotine
Furans:
- Furan (C4H4O)
- Furosemide
Thiophenes:
- Thiophene (C4H4S)
- Thiobarbituric Acid
Pyrimidines:
- Pyrimidine (C4H4N2)
- Allopurinol
Pyrroles:
- Pyrrole (C4H5N)
- Indole
Imidazoles:
- Imidazole (C3H4N2)
- Clotrimazole
Pyrrolidines:
1.Pyrrolidine (C4H9N)
Quinolines:
- Quinoline (C9H7N)
- Chloroquine
These examples represent just a fraction of the wide variety of heterocyclic compounds that exist, each with its own unique properties and applications in chemistry, medicine, and materials science. Heterocyclic compounds are essential in the development of pharmaceuticals, agrochemicals, and many other chemical products.
0 Comments